I was a bit alarmed Wednesday, November 10, afternoon when I discovered the water level in my MicroAquarium was alarmingly low. Was it knocked over and spilled or just overlooked when water should have been added? Evaporation is pretty significant and water is normally added two to three times per week.
Last week, November 3, I noticed small nematodes in the MicroAquarium. I wasn't sure what they were but over the week they had grown pretty significantly so I could identify them as nematodes (Rainis and Russell 1996). They were crawling along the bottom through the sediment looking for food.
Halteria sp. and Pinnularia sp. continue to be the predominant species observed. Other organisms I continue to see include Ostracods (seed shrimp) (Rainis and Russel 1996) one or two at a time, protozoa, rotifers, and algae.
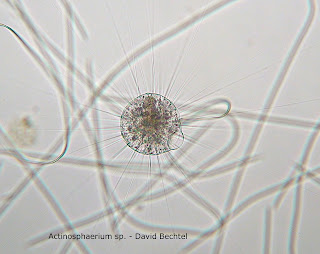
Below this entry I have attached photographs of Actinosphaerium sp. (Patterson 1996) and Pinnularia sp. (Canter-Lund and Lund 1995). I only observed Actinosphaerium on one occasion. What happened to them?
Citations:
Rainis KG, Russel BJ. 1996. Guide to Microlife. Danbury, CT: Franklin Watts.
Patterson DJ. 1996. Free-Living Freshwater Protozoa. London: Manson Publishing, Ltd.
Canter-Lund H, Lund JWG. 1995. Freshwater Algae. Bristol (England): Biopress Ltd.
Tuesday, November 16, 2010
Sunday, November 7, 2010
I've Got Protazoa On My Mind
Wednesday November 3, 2010 I finally identified Actinosphaerium sp., spotting two individuals, in my MicroAquarium and took a nice photograph that I plan to post next week. In the past I have misidentified Halteria sp. as Actinosphaerium within my blog so I will edit my earlier post to reflect this new knowledge.
Actinosphaerium are much larger than Halteria and move more slowly. They are very much less abundant also, with Halteria being one of the most predominant organisms in my MicroAquarium. Halteria sp. makes very rapid movements making it difficult to get a close up photo because as soon as one is in focus it jumps out of the frame. Below this post is a video I made Wednesday of a number of Halteria sp. (Patterson 1996) feeding on bacteria. The bacteria are consuming a dead rotifer. You can see how Halteria move in the video.
The carnivorous plant, Utricularia gibba L, has small gourd shaped traps spaced along its length. In one of the traps I could see a seed shrimp that appeared to be alive. The traps have some kind of fibers that let prey enter but close off the exit as kind of a flexible funnel where the outlet of the funnel expands but contracts once the prey has passed.
In one part of the MicroAquarium there appears to be a small green algae bloom. Many individuals of a green organism that appear to be fairly immobile where arrayed in a fan shape. I hope to identify them next week.
Citation:
Patterson DJ. 1996. Free-Living Freshwater Protozoa. London: Manson Publishing, Ltd.
Actinosphaerium are much larger than Halteria and move more slowly. They are very much less abundant also, with Halteria being one of the most predominant organisms in my MicroAquarium. Halteria sp. makes very rapid movements making it difficult to get a close up photo because as soon as one is in focus it jumps out of the frame. Below this post is a video I made Wednesday of a number of Halteria sp. (Patterson 1996) feeding on bacteria. The bacteria are consuming a dead rotifer. You can see how Halteria move in the video.
The carnivorous plant, Utricularia gibba L, has small gourd shaped traps spaced along its length. In one of the traps I could see a seed shrimp that appeared to be alive. The traps have some kind of fibers that let prey enter but close off the exit as kind of a flexible funnel where the outlet of the funnel expands but contracts once the prey has passed.
In one part of the MicroAquarium there appears to be a small green algae bloom. Many individuals of a green organism that appear to be fairly immobile where arrayed in a fan shape. I hope to identify them next week.
Citation:
Patterson DJ. 1996. Free-Living Freshwater Protozoa. London: Manson Publishing, Ltd.
Wednesday, November 3, 2010
Saturday, October 30, 2010
Week Three
On Friday October 22, 2010 a Beta Food Pellet was added to the microaquarium and remained near the top and right end. Specifically the food pellet is: "Atison's Betta Food" made by Ocean Nutrition, Aqua Pet Americas, 3528 West 500 South, Salt Lake City, UT 84104. Ingredients: Fish meal, wheat flower, soy meal, krill meal, minerals, vitamins and preservatives. Analysis: Crude Protein 36%; Crude fat 4.5%; Crude Fiber 3.5%; Moisture 8% and Ash 15%.
On Tuesday October 26 I set out to get a better feel for the the populations of different organisms in my microaquarium. I set the microaquarium on the microscope stage and began scanning across it and up and back, etc. until I had a feel for the distribution of organisms in the microaquarium. In the process of scanning I was distracted by a couple things happening in the microaquarium and paused for a closer look. On the bottom I observed a relatively large organism which I have tentatively identified as a seed shrimp. It was foraging in the bottom of the aquarium and moving quickly so it was difficult to keep track of or to get a photograph.
Near the right side of the microaquarium I observed several of the protozoa Coleps sp. feeding on something supported in the plant material. On close observation it appeared to be a dead rotifer but on closer observation I could see that they were feeding on the bacteria that were consuming the rotifer. You can see a video of the Coleps sp. feeding in the video just below this entry. Coleps sp. are distinguished by their scaly appearance and a few small barbs at the rear. Their bodies are composed of about ten cells. (Patterson 1996)
On completing my tour of the microaquarium I observed that there is a population boom in the microaquarium as a result of the addition of the food pellet. There was a halo of organisms around the pellet, mostly relatively small to medium size organisms. The most abundant organism observed was Halteria sp.
Throughout the microaquarium I observed the diatom Pinnularia evenly distributed and in relatively low abundance. Organisms were most abundant at the food pellet and among the plants and near the bottom. The center of the aquarium was relatively barren except for the Pinnularia. The larger organisms, the rotifers, were in the area around the plants and near the bottom generally. The largest organism observed was the seed shrimp foraging on the bottom.
Citation:
Patterson DJ. 1996. Free-Living Freshwater Protozoa. London: Manson Publishing, Ltd.
On completing my tour of the microaquarium I observed that there is a population boom in the microaquarium as a result of the addition of the food pellet. There was a halo of organisms around the pellet, mostly relatively small to medium size organisms. The most abundant organism observed was Halteria sp.
Throughout the microaquarium I observed the diatom Pinnularia evenly distributed and in relatively low abundance. Organisms were most abundant at the food pellet and among the plants and near the bottom. The center of the aquarium was relatively barren except for the Pinnularia. The larger organisms, the rotifers, were in the area around the plants and near the bottom generally. The largest organism observed was the seed shrimp foraging on the bottom.
Citation:
Patterson DJ. 1996. Free-Living Freshwater Protozoa. London: Manson Publishing, Ltd.
Tuesday, October 26, 2010
Sunday, October 24, 2010
Starting to Identify Some of the Inhabitants
This week I explored my MicroAquarium at a microscope that has a camera attached so you can take photos and also observe on a desktop screen instead of peering through the microscope. I photographed several creatures and made a short video of a Licanidae rotifer (Thorp and Covich 1991) which can be viewed below. Other creatures I have identified include a rotifer Pterodina (Ward and Whipple 1918) and a diatom Pinnularia (Canter-Lund and Lund 1995). Pinnularia stack together and I observed a stack of two and a stack of four. I will try to attach photos of these and others next week when I have identified more.
I spent a lot of time learning to use the equipment and editing my photos this week. Next week I plan to spend more time trying to get a sense of the populations of creatures in my MicroAquarium and identifying the creatures I have photographed this week.
Citations:
Thorp JH, Covich AP ed. 1991. Ecology and Classification of North American Freshwater Invertebrates. San Diego: Academic Press, Inc.
Ward HB, Whipple GC. 1918. Fresh-water Biology. New York: John Wiley & Sons, Inc.
Canter-Lund H, Lund JWG. 1995. Freshwater Algae. Bristol (England): Biopress Ltd.
I spent a lot of time learning to use the equipment and editing my photos this week. Next week I plan to spend more time trying to get a sense of the populations of creatures in my MicroAquarium and identifying the creatures I have photographed this week.
Citations:
Thorp JH, Covich AP ed. 1991. Ecology and Classification of North American Freshwater Invertebrates. San Diego: Academic Press, Inc.
Ward HB, Whipple GC. 1918. Fresh-water Biology. New York: John Wiley & Sons, Inc.
Canter-Lund H, Lund JWG. 1995. Freshwater Algae. Bristol (England): Biopress Ltd.
Friday, October 15, 2010
MicroAquariumTM Set Up
Wednesday October 13, 2010 I set up my MicroAquarium. A MicroAquarium is made of two rectangular pieces of glass approximately 5 cm X 8 cm fastened together parallel to each other with watertight adhesive on three sides so the space between is about 3 mm creating a small aquarium. Through the fourth side, the open side, water containing microorganisms is injected. When the MicroAquarium is carefully laid on its side without tipping the end, surface tension keeps the water from spilling so it can be safely placed on the microscope stage to observe the microorganisms.
First I labeled my MicroAquarium to distinguish it from all the other lab participants' and secured a stand and cover to keep it safe and erect in the lab between the observations I will make at least once weekly. Using a pipet I extracted water from a source provided in the lab, first pulling water from the bottom, including some soil, to fill the MicroAquarium one third, then equal portions from the top and middle of the source. I also added two plants.
On the left side of the MicroAquarium I placed the plant: Fontinalis sp. Moss. Collection from: Natural spring. at Carters Mill Park, Carter Mill Road, Knox Co. TN. Partial shade exposure. N36 01.168 W83 42.832. 10/10/2010.
And on the right side: Utricularia gibba L. Flowering plant. A carnivous plant. Original material from south shore of Spain Lake (N 35o55 12.35" W088o20' 47.00), Camp Bella Air Rd. East of Sparta Tn. in White Co. and grown in water tanks outside of greenhouse at Hesler Biology Building. The University of Tennessee. Knox Co. Knoxville TN.
My water source came from a Plastic Bird Bath pool. 0.9 mile from Fountain City Pond on Fountain Rd. Knox Co. Knoxville TN Partial shade exposure N 36o02.249' W083o55.999' 1121 ft 10/10/2010. (McFarland 2010)
After setting up the MicroAquarium I made some observations with the microscope. I observed a few round organsims that had long cilia and were mostly motionless but would occasionally move explosively. Other much smaller round organisms appeared to rotate and move around at a fairly rapid pace. Cigar shaped organisms moved slowly through the water. Another organism with a long flagellum and body shaped like a top was seen. Also, I saw long thin green stationary organisms which appear to be one cell thick. These had regular divisions across them, probably the cell walls.
Citations:
McFarland, Ken. 4 October 2010. Botany 111 2010. An inquiry into the dynamic microorganisms in our environment. 15 October 2010. <http://botany1112010.blogspot.com/2010_10_01_archive.html>.
First I labeled my MicroAquarium to distinguish it from all the other lab participants' and secured a stand and cover to keep it safe and erect in the lab between the observations I will make at least once weekly. Using a pipet I extracted water from a source provided in the lab, first pulling water from the bottom, including some soil, to fill the MicroAquarium one third, then equal portions from the top and middle of the source. I also added two plants.
On the left side of the MicroAquarium I placed the plant: Fontinalis sp. Moss. Collection from: Natural spring. at Carters Mill Park, Carter Mill Road, Knox Co. TN. Partial shade exposure. N36 01.168 W83 42.832. 10/10/2010.
And on the right side: Utricularia gibba L. Flowering plant. A carnivous plant. Original material from south shore of Spain Lake (N 35o55 12.35" W088o20' 47.00), Camp Bella Air Rd. East of Sparta Tn. in White Co. and grown in water tanks outside of greenhouse at Hesler Biology Building. The University of Tennessee. Knox Co. Knoxville TN.
My water source came from a Plastic Bird Bath pool. 0.9 mile from Fountain City Pond on Fountain Rd. Knox Co. Knoxville TN Partial shade exposure N 36o02.249' W083o55.999' 1121 ft 10/10/2010. (McFarland 2010)
After setting up the MicroAquarium I made some observations with the microscope. I observed a few round organsims that had long cilia and were mostly motionless but would occasionally move explosively. Other much smaller round organisms appeared to rotate and move around at a fairly rapid pace. Cigar shaped organisms moved slowly through the water. Another organism with a long flagellum and body shaped like a top was seen. Also, I saw long thin green stationary organisms which appear to be one cell thick. These had regular divisions across them, probably the cell walls.
Citations:
McFarland, Ken. 4 October 2010. Botany 111 2010. An inquiry into the dynamic microorganisms in our environment. 15 October 2010. <http://botany1112010.blogspot.com/2010_10_01_archive.html>.
Subscribe to:
Comments (Atom)